Researchers identify reasons for failure of animal heart tissue used in replacement heart valves for treatment of aortic stenosis
Study will result in pre-screening of tissues and prevention of failure of bioprosthetic valves going forward
Researchers based in AMBER, Trinity College Dublin have identified reasons for the failure of animal heart tissue i.e. bioprosthetic valves used in the treatment of Aortic Stenosis which will result in pre-screening and prevention of failure of bioprosthetic valves going forward. This research was recently published in Acta Biomaterialia.
Aortic Stenosis (AS) is the narrowing and stiffening of the aortic valve, which restricts blood flow from the heart to the rest of the body. Severe AS is a life-threatening condition which has a 2-year mortality rate of close to 50% if left untreated.
Thankfully minimally invasive treatments are available for Aortic stenosis involving the implantation of a bioprosthetic valve, a biological prosthesis with valve leaflets made from animal heart tissue (pericardium). Animal bioprosthetic valves are preferred over mechanical valves due to the reduced risk of blood clots and the elimination of the need for long-term anticoagulation medication.
However, these bioprosthetic valves frequently succumb to failure due to regurgitation, when the valve does not close properly and causes blood to flow backwards or abnormal narrowing (stenosis) caused in part by calcification and structural damage. The cause of this failure has divided researchers for a while where some have suggested that valve leaflet material failure such as tearing is due to calcification and others have suggested it is due to damage.
Lead Researcher, Professor Tríona Lally of AMBER, the Research Ireland Centre for Advanced Materials and BioEngineering said of the study: “This research explores the relationship between calcification and structural damage in heart valve leaflets. We show a synergistic effect of these factors and that leaflet material (porcine pericardium) fibre patterns play a significant role in early failure of these materials. Within our lab, we can pre-screen these tissues to identify their fibre patterns which should mean we can better prevent failure of bioprosthetic valves going forward.”
To establish these findings the research team lead by Tríona, Professor in Biomedical Engineering within the Department of Mechanical, Manufacturing & Biomedical Engineering, Trinity College Dublin, with Enterprise Partnership funded PhD student Luke Guerin and colleagues from Boston Scientific Galway, first, exposed animal heart tissue, in this instance porcine pericardium (PP) to either in vitro calcification (investigating calcification alone) or cyclic bulge loading in saline (investigating structural damage alone).
Subsequently, the animal heart tissue was simultaneously calcified and cyclically loaded for 30 million cycles. Simultaneous calcification and loading led to dramatically increased calcification and structural damage, including tissue rupture. Fibre architecture was found to affect rupture location and dramatically affect the rate of rupture.
This finding has implications for future bioprosthetic valve leaflet anti-calcification strategies, where tissue mechanics influenced by the underlying tissue fibre architecture should be considered to minimise both structural damage and calcification.
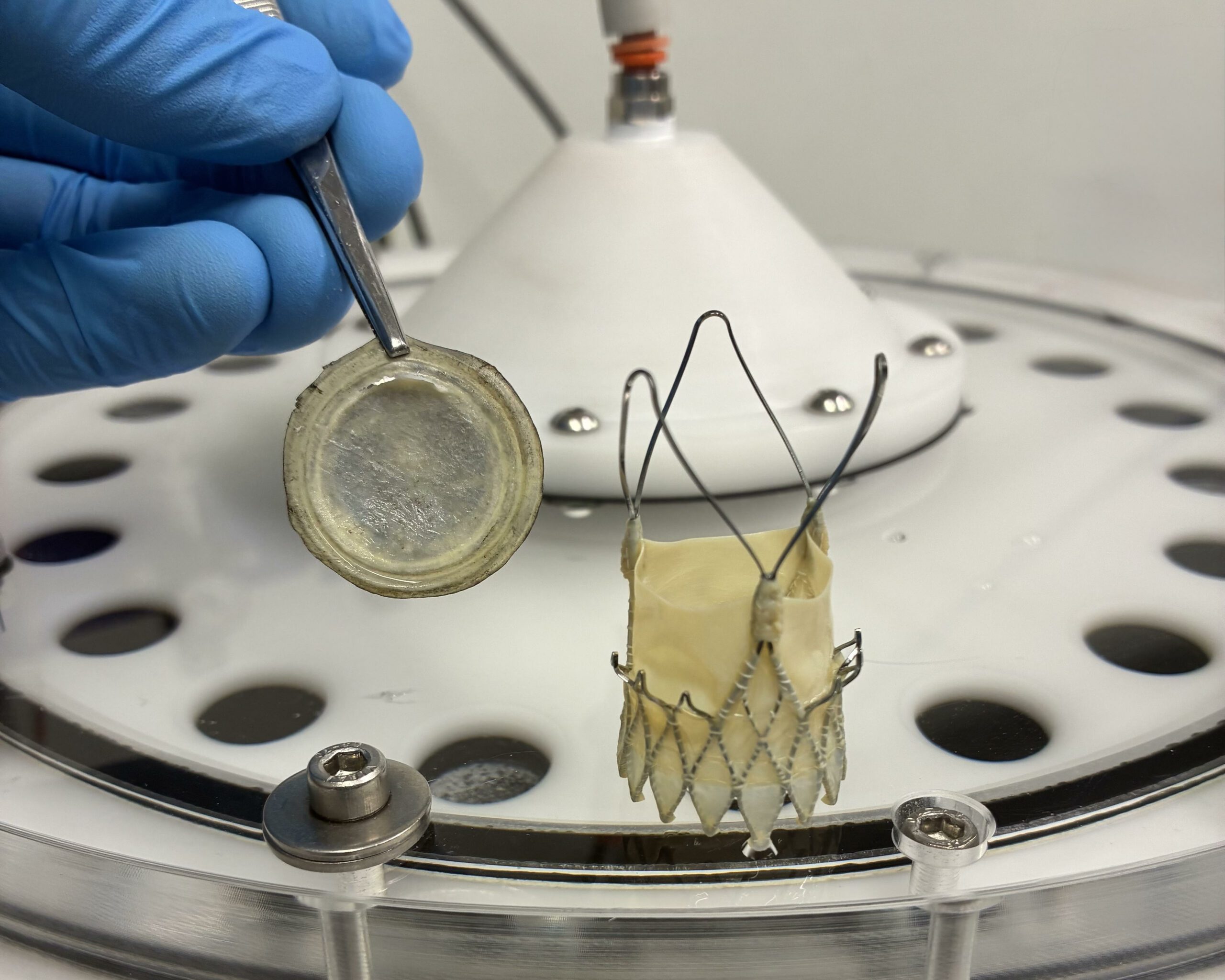
Sample of the bioprosthetic valve
AMBER has a strong emphasis on collaboration. Central to AMBER’s research remit are collaborative projects performed with industry partners, and working with academic, industry and wider stakeholder on international and national research programmes.
Get in touch